
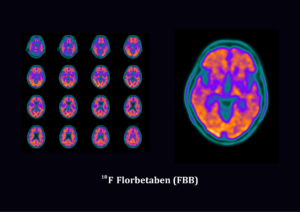
Florbetaben is not approved in Australia for clinical routine. Florbetaben is available under the special access scheme for single patients and for clinical trials. In Europe and US florbetaben is approved as Neuraceq®, which is a radioactive diagnostic agent indicated for Positron Emission Tomography (PET) imaging of the brain to estimate beta amyloid neuritic plaque density in adult patients with cognitive impairment who are being evaluated for Alzheimer’s disease (AD) and other causes of cognitive decline.
PI-2620 was discovered in a research collaboration with Life Molecular Imaging and AC Immune. Life Molecular Imaging has the exclusive worldwide license for the research, development and commercialization of Tau-PET tracers generated within the discovery program. PI-2620 is currently under investigation in several clinical studies as a targeted radiopharmaceutical for the detection of Tau deposits in the human brain. PI-2620 is not approved in Australia for clinical routine but is available for clinical trials.
Life Molecular Imaging (LMI, formerly Piramal Imaging) was formed in 2012 with the acquisition of the molecular imaging research and development portfolio of Bayer Pharma AG. It is now part of the Alliance Medical Group (a member of the Life Healthcare Group) offering an integrated business including research and development laboratories, a network of cyclotrons, radiopharmacies and imaging facilities. By developing novel PET tracers for molecular imaging, LMI is focusing on a key field of modern medicine. The organization strives to be a leader in the Molecular Imaging field by developing innovative products that improve the early detection and characterization of chronic and life-threatening diseases, leading to better therapeutic outcomes and improved quality of life. Please visit https://life-mi.com.
[contact-form-7 id=”714″ title=”Update Form”]